Measuring the novelty of new drugs – Healthcare Economist
Measuring the novelty of new drugs
Measuring the novelty of new drugs is a crucial aspect of drug discovery and development. It helps researchers and regulatory agencies assess the potential benefits and risks associated with a new drug candidate. Several methods and criteria are used to evaluate the novelty of new drugs:
- Chemical Structure: One of the most basic ways to assess the novelty of a drug is by analyzing its chemical structure. If the drug has a unique chemical structure or belongs to a new class of compounds, it is considered more novel. This can be determined through chemical synthesis and structural analysis.
- Mechanism of Action: Understanding how a drug works at the molecular level is essential. A drug that targets a novel pathway or has a unique mechanism of action is considered more innovative. This often involves studying the drug’s interactions with specific molecular targets, such as proteins or enzymes.
- Therapeutic Target: The target of a drug, such as a specific protein or receptor, can also determine its novelty. Drugs that target previously unexplored or validated targets are considered more novel. Validating new targets often involves extensive research and experimentation.
- Clinical Indications: The intended clinical use of a drug can influence its novelty. Drugs that address unmet medical needs or offer significant improvements over existing treatments are considered more innovative. This assessment may consider factors like efficacy, safety, and patient outcomes.
- Preceding Drugs: Comparing the new drug to existing drugs within the same therapeutic class can help gauge its novelty. If the drug offers advantages over existing treatments, such as improved efficacy, reduced side effects, or better patient compliance, it may be considered more innovative.
- Regulatory Designations: Regulatory agencies, such as the U.S. FDA, may grant special designations to drugs that demonstrate significant novelty. These designations, such as “Orphan Drug” status or “Breakthrough Therapy” designation, indicate that the drug addresses specific unmet needs or has shown exceptional promise in clinical trials.
- Scientific Publications: The number and quality of scientific publications related to a drug candidate can also reflect its novelty. If a drug has been extensively studied and published in reputable journals, it may be considered more innovative.
- Intellectual Property: Patents granted for a drug candidate can provide insights into its novelty. A unique or novel chemical composition or method of treatment can be an indication of innovation.
- Market Analysis: Market analysts and experts often evaluate the competitive landscape and market potential of a new drug. A drug that is expected to disrupt or significantly impact the market may be considered more novel.
- Real-world Evidence: As a drug enters the market and is used in clinical practice, real-world data can provide insights into its novelty. Observations of patient outcomes, adverse events, and long-term effects can contribute to the assessment of a drug’s uniqueness.
Measuring the novelty of new drugs is a multifaceted process that combines scientific, regulatory, and market-oriented evaluations. It is essential to consider various aspects to make informed decisions about drug development, regulatory approvals, and market access. Additionally, the level of novelty can impact a drug’s patent protection and market exclusivity, further influencing its commercial success.
All of us want new, improved medicines. Drugs that provide incremental benefits (often called “me too” drugs) are good, but truly revolutionary therapies are even better. However, how does one separate how “novel” a treatment is? Is there a way to quantify this?
In fact there is. One way to do this is based on the treatment’s molecular structure. For instance, the Tanimoto distance measures the the fraction of chemical features that are shared by the two chemical compounds. Nikolova et al. 2004 summarizes this approach which is calculated as follows:
Krieger, Li and Papanikolau 2022 use this method to quantify how drug development for novel drugs has much more positive informational spillovers on the drug development than incremental drugs. This paper provides an example of an application of Tanimoto distance below.
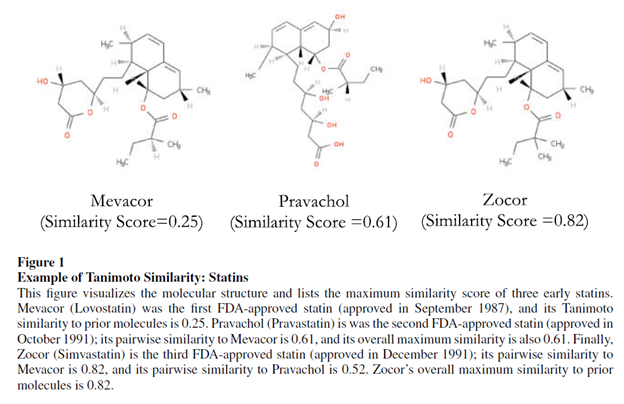
Using the Tanimoto distance as a measure of novelty, the authors find the following:
…novel drug candidates are less likely to obtain FDA approval but are based on more valuable patents. Consistent with a simple model of costly external finance, we show that a positive shock to firms’ net worth leads firms to develop more novel drugs. This suggests that even large firms may behave as though they are risk averse, reducing their willingness to investment in potentially valuable radical innovation.
You can read the full paper here.